面對日益嚴重的細菌耐藥性和超級細菌的不斷產(chǎn)生,,開發(fā)新型抗生素和尋找全新作用機制的抗菌藥物是有效應對這一全球公共衛(wèi)生危機的重要策略,。目前,,臨床試驗中絕大多數(shù)抗菌藥物是對以前上市的抗生素的修飾和改造,,其中包括天然產(chǎn)物——截短側(cè)耳素。截短側(cè)耳素是一類具有駢三環(huán)骨架的二萜化合物,,具有較好的抗菌活性且結(jié)構(gòu)獨特,、作用機制新穎,,不易產(chǎn)生耐藥性,,具有很好的開發(fā)前景,但該類藥物存在口服生物利用度,、代謝穩(wěn)定性的研發(fā)難點,。
近日,我院獸醫(yī)獸藥研究所獸藥研發(fā)團隊在前期探索吡咯并嘧啶骨架對截短側(cè)耳素的活性和水溶性影響的基礎上(Eur. J. Med. Chem.2019, 162, 194-202),,采取藥物結(jié)構(gòu)優(yōu)化中經(jīng)典的骨架躍遷策略,,在截短側(cè)耳素C-14側(cè)鏈引入親水基團取代的喹唑啉酮骨架。體外抗菌活性結(jié)果顯示,,4(3H)-喹唑啉酮骨架可以有效提高抗革蘭氏陽性菌活性,,抗MRSA的MIC為0.25μg/mL,與上市藥物泰妙菌素(MIC=0.5μg/mL)相當,。該研究進一步采取骨架躍策略遷得到化合物23,,其在體內(nèi)外抗菌活性及體外細胞毒性和代謝穩(wěn)定性均好于上市動物專用藥物—延胡索酸泰妙菌素。相關(guān)研究結(jié)果以“Antibacterial activity evaluation of pleuromutilin derivatives with 4(3H)-quinazolinone scaffold against methicillin-resistant Staphylococcus aureus”為題在線發(fā)表在《歐洲藥物化學(European Journal of Medicinal Chemistry)》,。該研究為后期深入研究截短側(cè)耳素類抗菌藥物并篩選先導化合物提供了重要基礎,。
該研究獲得重慶市自然科學基金面上項目支持。
全文鏈接:https://doi.org/10.1016/j.ejmech.2022.114960.
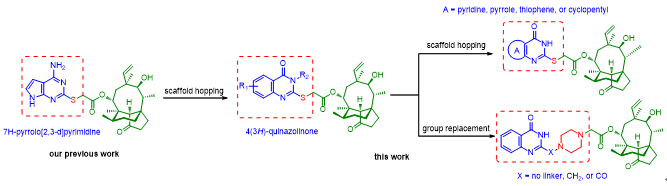
Figure 1. Design of pleuromutilin derivatives as novel antibacterial agents via combining 4(3H)-quinazolinone scaffold to the C-14 side chain.
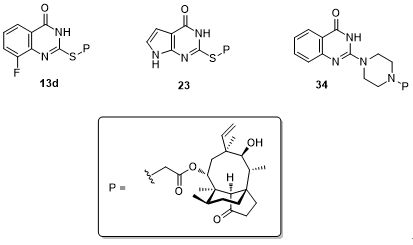
Figure 2. The structures of compounds 13d, 23 and 34
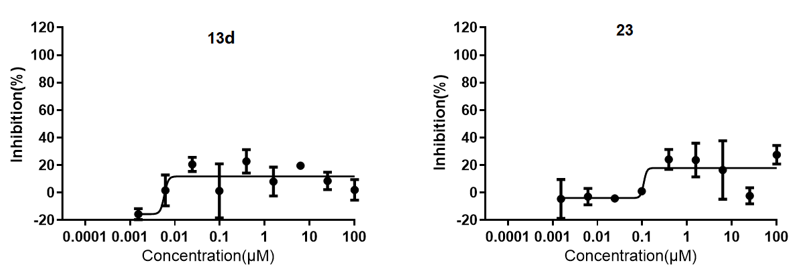
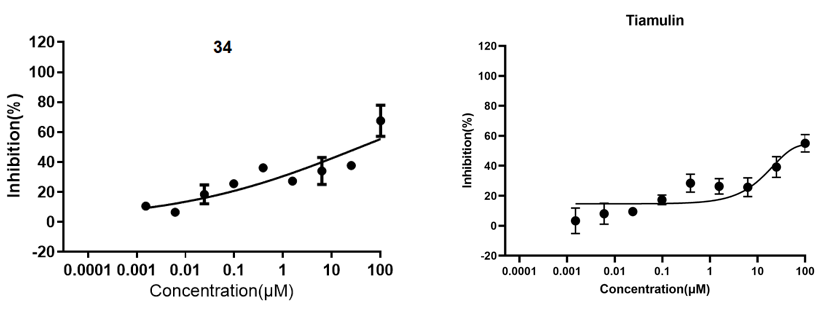
Figure 3. Cytotoxicity of compounds 13d, 23, 34, and tiamulin for RAW264.7 cells.
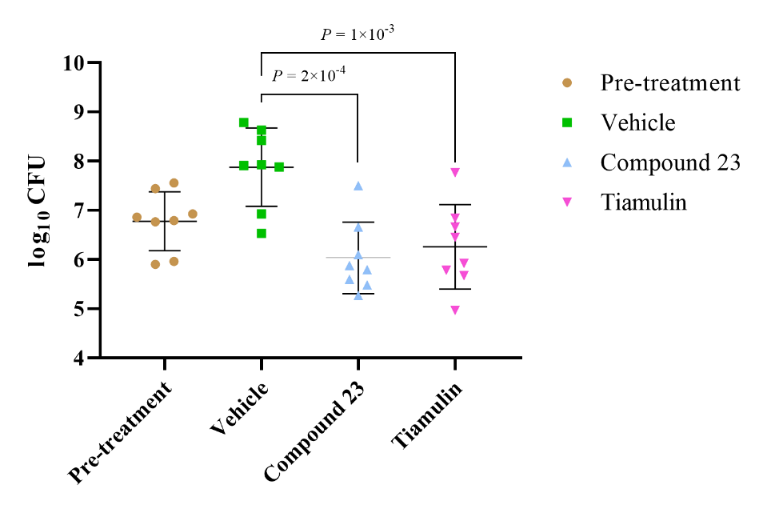
Figure 4. Efficacy of 23 at a dose of 50 mg/kg in a neutropenic mouse thigh infection model. Mice were treated with vehicle and test compounds intraperitoneally and bacterial counts were determined after 26 h treatment. Data are displayed mean±s.d.;n = 8 thighs from 4 mice examined over 2 experiments and two-tailed unpaired Welch’s t-test was used for statistical analysis.